Nov. 8, 2021
Visualization of ACE2, a Coronavirus Target Protein, by NanoSIMS Imaging
[Abstract]
Toray Research Center, Inc. has succeeded in visualizing the coronavirus target protein ACE2 at the cellular level by combining our uniquely developed labeled substance for secondary ion mass spectrometry with the NanoSIMS 50L, which enables mass imaging with high spatial resolution.
[Background]
The new coronavirus infection (COVID-19) that occurred in 2019 has since spread around the world, and even with the current progress in vaccination, the spread of the infection cannot be stopped. Angiotensin-converting enzyme II (ACE2) is the target of SARS-CoV-2 when it infects cells, and the distribution of ACE2 expression can be determined by immunostaining using antibodies against ACE2. But it is not easy to elucidate the expression distribution at the cellular level using conventional methods due to the influence of residual blood in the tissue and the resolution of imaging.
SIMS, which is used for mass imaging, is an analytical method that directly detects elements contained in a solid sample by detecting ions (secondary ions) emitted from the surface by sputtering during ion beam (primary ions) irradiation to the solid surface. In particular, the NanoSIMS 50L combines an ion beam with a probe diameter of approximately 50 nm and a mass spectrometer with high transmittance, enabling simultaneous analysis of up to seven elements at the highest spatial resolution (<50 nm) for mass imaging. In order to maximize the performance of the NanoSIMS 50L, we have been working on the synthesis of various labeled materials that efficiently emit secondary ions with the ion beam.
[Achievement]
This time, we have succeeded in clarifying the localization of ACE2 in lung tissue at the cellular level. By utilizing our uniquely developed label-modified antibody and maximizing the performance of NanoSIMS 50L, we were able to visualize ACE2 at high spatial resolution.
The detailed analysis results are shown below.
Figure 1 shows the distribution image of ACE2 in rat lung tissue. The fluorescence micrograph shown on the left is an immunostaining image of lung tissue. Although the space corresponding to the bronchial lumen and the lung tissue can be clearly distinguished, it is difficult to distinguish the non-specific fluorescence emitted by residual blood from that of the labeled material. Moreover, since the resolution of the fluorescence microscopy is limited, the distribution of ACE2 cannot be clarified only from the left figure.
On the other hand, the right figure shows the area indicated by the square in the left figure, which was measured by NanoSIMS to visualize the secondary ions derived from the labeled antibody. It can be seen that ACE2, indicated by the white signal, is abundantly distributed in the cell membrane on the luminal side of the bronchial epithelial cells. Since the bronchial lumen is the space through which expiratory air containing the virus passes, and the luminal surface of the bronchial epithelial cells is the first place to which the virus inhaled into the lung adheres, this data shows that the virus can easily infect cells by using ACE2 as a target.
As shown in this analysis example, the greatest feature of our novel method using labeled material and NanoSIMS is that it can visualize target molecules in biological tissues with high spatial resolution without being affected by residual blood or other components.
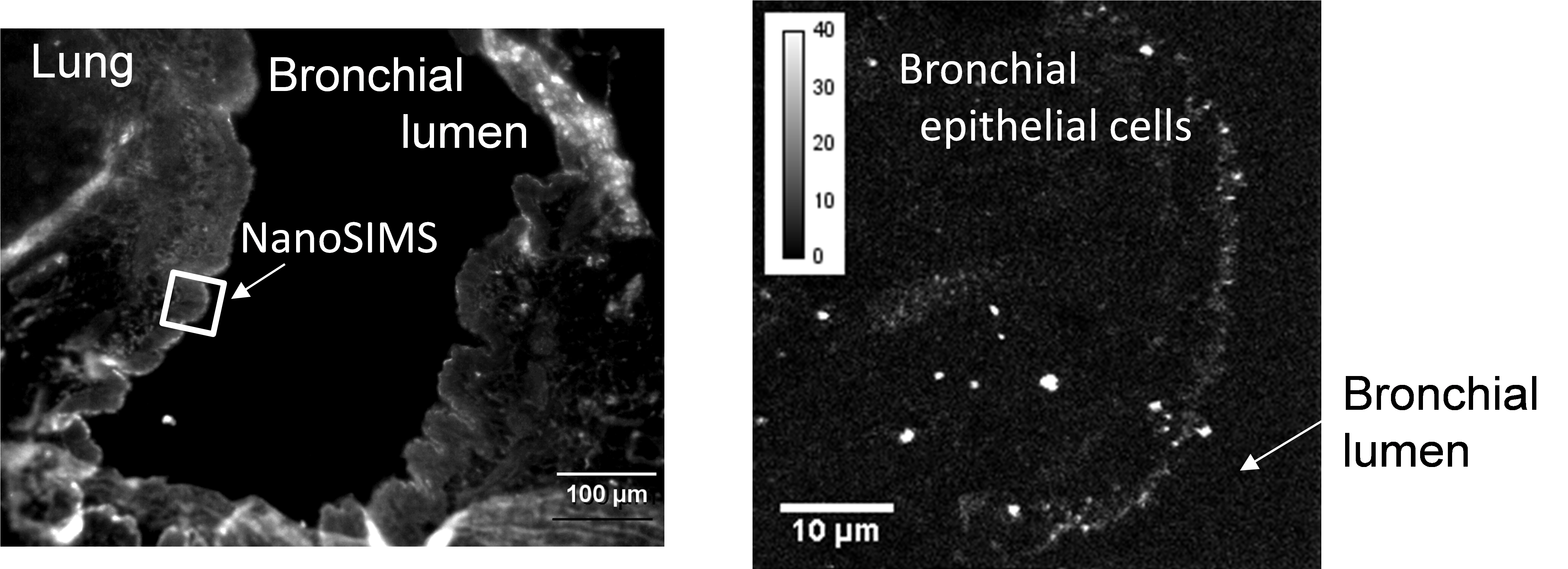
Fig. 1 Images of ACE2 distribution in rat lung tissue
(Left) Wide area image by fluorescence microscopy,
(Right) High resolution image of the “measurement area” of the left image by NanoSIMS.
[Future Prospects]
This result clearly shows that the possibility of this method combining NanoSIMS and the labeled material in the life science field will be greatly expanded. We are currently constructing a measurement system to establish the so-called intracellular localized imaging, which clarifies not only the tissue distribution of drugs administered to animals, but also the localization of proteins and nucleic acids to which organelles in cells. This method will enable, for example, the evaluation of nuclear localization of nucleic acid drugs and recycling of antibody drugs, which will increase the certainty of drug discovery research and contribute to shortening the drug development period. We will continuously develop our technology so that we can bring the latest analytical technology to the field of drug development as soon as possible.
[Contact]
For inquiries regarding the contents of this press release, please contact by following inquiry form.
https://cs2.toray.co.jp/contact/k0090.nsf/inquiry?Openform&&en